During the first part of the project at the University of Palermo, a module on “Nanotechnology in solar energy conversion” have been designed and tested. In the second phase, currently running, about 20 classes and 400 students are involved on the same module and, in addition, the photocatalysis module, developed by the Polish colleagues of Jagiellonian University, is also being tested.
Based on the fact that both modules make use of TiO2 (titanium dioxide), it was decided to hold a meeting among all teachers of the different CoLs in order to discuss the properties of this popular and important compound. During the meeting the importance of TiO2 as photocatalyst was highlighted by stressing the chemical and physical features that make this substance particularly interesting.
TiO2 has been used as a white pigment in paints since 1916 and its main applications are still as a pigment – in paints, plastics and rubber, paper, inks, cosmetics and pharmaceuticals. Indeed, of the six million tones produced globally each year, 99% is used in pigments. We use products containing titania every day, from the moment we squeeze the toothpaste tube in the morning, to when we reach for a piece of chewing gum, slap on sun cream, or swallow a tablet.

TiO2 is invariably present in creams and solar screens
As photocatalyst it was used for the first time in Japan in the 1970s and it is still involved today in many research areas, such as dye-sensitized solar cells (DSSCs), self-cleaning glasses, antimicrobial products, water and air purification and water splitting.
Photocatylic properties of TiO2 arise from its semiconductor properties, gaining free electrons only under certain conditions, when irradiated or heated. Differently from conductors, where electrons are free to move from the valence band to the conduction band, in semiconductors there is an energy gap between these two states that can be overcome if the material absorbs radiation with sufficient energy. In fact, when titania (band gap of about 3 eV) is exposed to UV light, an electron is promoted in the conduction band, leaving behind a hole. Titania is widely used because it is chemically and biologically inert, mechanically robust, inexpensive, and highly active.
During the meeting teachers realized that in both modules they were taking advantage of titania photocatalytic properties in different ways. In DSSC cell, the TiO2 layer on the anode works as electron transporter. Indeed, free electrons available in the light sensitized dye were transferred in the TiO2 conduction band due to the energetic proximity of the bands. In the case of self-cleaning glasses (experiments involved in some steps of the catalysis module), the generated electron/hole pair is able to degrade organic compounds. In particular, water neutralizes the holes generating hydroxyl radicals .OH, oxygen accepts electrons generating superoxide radicals. Both these species are strong oxidizing agents thus capable of degrading organic substances. It is worth noticing that the efficiency of the above processes can be enhanced by maximizing the TiO2/substrate contact. For this reason, TiO2 nanoparticels are used with the aim of either maximizing dye/conduction band electron transfer or the transfer of electrons from conduction band to oxygen.
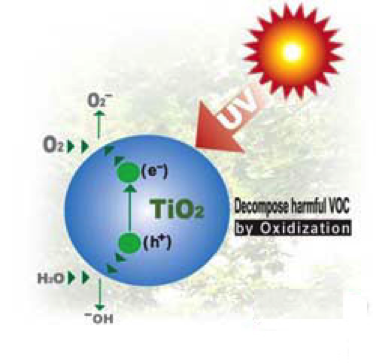
Electron/hole pair in TiO2 produced by UV light.
The species can react with oxygen and water to produce strong oxidizing agents.








